Blame for the opioid crisis in the U.S. often falls squarely on pharmaceutical companies, pharmacies or rogue prescribers — like the Virginia doctor who prescribed more than half a million opioid doses in two years.
But the whole story is more complicated, and it implicates a large portion of health care providers. Research shows that many doctors, nurse practitioners and physician assistants across the nation have oversupplied patients with opioids, spurring a national crisis that each year claims tens of thousands of lives.
“This isn’t just a story about rogue prescribers and pill mills,” says Caleb Alexander, co-Director of the Center for Drug Safety and Effectiveness at the Johns Hopkins School of Public Health. “A much broader swath of the medical profession is responsible for the oversupply of opioids in clinical practice.”
“Physicians are people, too”
Prescription painkillers have a high risk of abuse — across the literature, rates of misuse average between 21% and 29%. Research indicates that as of 2013, more than 2 million people in the U.S. had prescription opioid-related opioid use disorder.
Prescription opioids can also pave the way for illegal drugs like heroin. Eighty percent of people who have used heroin have previously misused prescription opioids, according to an August 2013 Center for Behavioral Health Statistics and Quality Data Review analyzing survey data collected from 2002 to 2011.
About 233.7 million opioid prescriptions were filled each year on average from 2006 to 2017, according to a March 2019 study in JAMA Network Open that looked at opioid prescriptions filled in retail pharmacies across the U.S.
Although the average amount of opioids prescribed per person declined over this period, the average prescription duration increased, this study found. So did the number of prescriptions written for a month or longer.
Research shows that a substantial share of the supply is not coming from high-volume prescribers or “pill mills.” One paper, published in Addiction in November 2017, analyzes 44.2 million prescription opioid pharmacy claims and finds low-volume prescribers were a major driver of the opioid supply.
Prescribers at the top-fifth percentile of total opioids distributed were more likely to give patients higher doses, longer supplies, more prescriptions, and greater overall amounts of opioids. But prescribers who make up the lower 95th percentile still account for a large share of the prescriptions given to high-risk patients. Low-volume prescribers account for 15% to 29% of overall amounts of opioids prescribed and 18% to 56% of opioid prescriptions received by high-risk patients.
“We found that a small number of prescribers account for a majority of opioids prescribed,” says Alexander, lead author of the paper. He adds, “A substantial proportion of patients who were using high volumes of opioids intersect with low-volume prescribers.”
The finding that some physicians consistently prescribe more opioids than others indicates that “when it comes to the receipt of opioids, it’s not just about who you are, it’s about who you see,” Alexander says. “The same person going to two different doctors may end up with wildly different supplies of opioids. And this speaks to the lack of standardization of care.”
A March 2017 research letter published in JAMA Internal Medicine looks at trends among Medicare Part D prescribers — a group of over 800,000 physicians prescribing opioids to people who use Medicare to buy prescription drugs. Opioid prescriptions are more common in certain specialties, but general practitioners dominate prescribing, according to the letter.
“Medicare opioid prescribing is distributed across many prescribers and is, if anything, less skewed than all drug prescribing,” the authors conclude. “High-volume prescribers are not alone responsible for the high national volume of opioid prescriptions.”
Another study published in October 2018 in Health Services Research corroborates this evidence and points to another prescribing trend in Medicare data — nurse practitioners and physician assistants account for the largest increases in quantity of opioid prescriptions written between 2006 and 2012.
“There’s a lot more prescribing of opioids by nurses and physician assistants, where they are state-by-state allowed to prescribe them,” says Sarah Axeen, the study’s author and assistant professor of research in the department of emergency medicine at the Keck School of Medicine of USC. “That hasn’t been highlighted in the same way [as physicians’ prescribing practices].”
Another paper, published in August 2019 in JAMA Network Open, analyzes the relationship between the time of day at which primary care appointments occur and opioid prescribing patterns.
Hannah T. Neprash and Michael L. Barnett analyzed electronic medical records from 678,319 appointments in 2017 for 642,262 patients with a painful condition and the 5,603 doctors who treated them. The researchers found that the likelihood that a physician’s patient would receive an opioid prescription rather than non-opioid pain medication or physical therapy increased by 33% as the day went on, and by 17% as appointments ran late.
“Physicians are people, too,” says Jason Doctor, professor and chair of the department of health policy and management at the University of Southern California’s Sol Price School of Public Policy, who was not an author on the paper. “They’re not always making rational decisions in the best interest of the patient, and sometimes they’re affected by the environment, their work situation. Dealing with risky drugs like opioids — that could be a problem.”
State of emergency
The medical community and media sometimes portray emergency departments as a key source of prescription opioids. Research complicates that narrative, too. A paper in Annals of Emergency Medicine from June 2018 finds that the majority of growth in opioid prescriptions nationally from 1996 to 2012 can be traced to office visits and refills of previously prescribed opioids, rather than prescriptions given in the emergency department.
A paper forthcoming in the American Journal of Emergency Medicine finds that in recent years, emergency departments have started prescribing fewer opioids and more non-opioid painkillers, such as non-steroidal anti-inflammatory drugs. Over the six years studied, the rate of opioid prescriptions given to patients in pain who went to two urban emergency departments and received some form of painkiller decreased from 37.76% to 13.29% between 2012 and 2018.
Within emergency departments, opioid prescribing can vary drastically from doctor to doctor. In a February 2017 New England Journal of Medicine paper, researchers looked at Medicare data from 2008 to 2011 for a random sample of 377,629 people who had visited an emergency department and received a range of diagnoses, including chest pain, vertigo, fatigue and high blood pressure. Whether patients with similar diagnoses at the same hospital received opioids depended on which physician they saw.
“Within individual hospitals, rates of opioid prescribing varied widely between low-intensity and high-intensity prescribers (7.3% vs. 24.1%),” the authors find. These differences in turn are linked to varying risk of long-term opioid use. Patients who were treated by high-intensity prescribers had significantly higher odds of developing long-term opioid use than those treated by low-intensity prescribers.
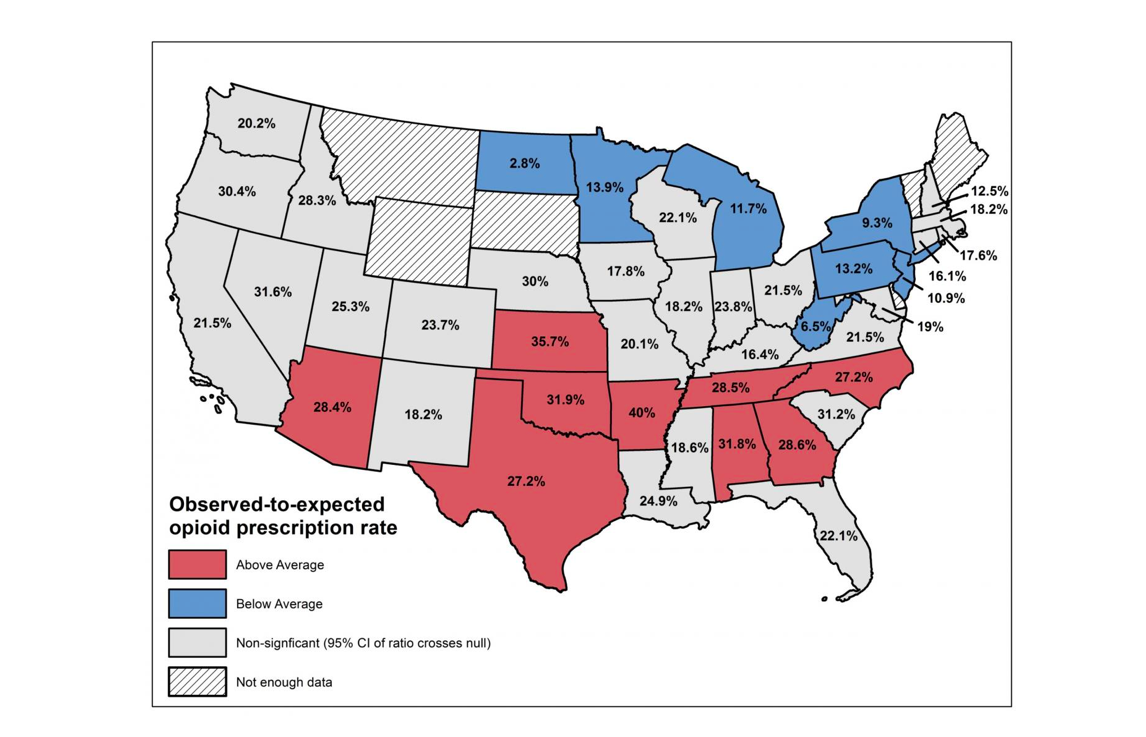
The opioid epidemic in hindsight
In 1980, Hershel Jick and Jane Porter, a physician and graduate student at Boston University Medical Center’s Boston Collaborative Drug Surveillance Program, published a letter to the editor in the New England Journal of Medicine titled “Addiction Rare in Patients Treated with Narcotics.”
Jick and Porter looked at a sample of 11,882 patients who received at least one opioid while hospitalized at the eight medical institutions affiliated with the Drug Surveillance Program. They found that only four of these patients developed opioid addictions.
They conclude: “Despite widespread use of narcotic drugs in hospitals, the development of addiction is rare in medical patients with no history of addiction.”
The letter comes in at just 101 words. It became a heavily-cited piece of evidence in support of the safety of opioids. In June 2017, NEJM published another letter to the editor describing the influence of Jick and Porter’s letter over the next 37 years of opioid research.
The four authors of the 2017 letter identified and analyzed 608 citations of the letter in the academic literature. Their review finds that citations of the letter increased noticeably after the introduction of the brand-name opioid OxyContin in 1995. Additionally, 72.2% of all the citations used the letter as evidence supporting the rarity of addiction to opioids. And 80.8% of all papers citing the letter failed to mention that the patients were hospitalized when they were receiving opioids.
In other words, none of the patients were individuals receiving opioids on an outpatient basis, where it would have been harder to monitor potential misuse of the drugs.
“We believe that this citation pattern contributed to the North American opioid crisis by helping to shape a narrative that allayed prescribers’ concerns about the risk of addiction associated with long-term opioid therapy,” the authors of the 2017 paper conclude.
Jick maintains that he is not responsible for the way his letter was used to allay concerns about the safety of opioids.
“I didn’t even know about it,” Jick says, referring to widespread citation of the letter as evidence that opioids are safe. “No one called and asked me about it. But the only reference in the medical references was a letter to the editor that I wrote in 1980 that was restricted to hospitalized patients. And the eventual outcome was that dozens and dozens of doctors picked this up and misused it. And I didn’t even know about it. I really wrote something that was accurate, but was irrelevant to the disaster that’s going on now.”
Pain: the fifth vital sign
The 1980s also marked the start of the pain movement, which centers on the idea that patients have a right not to be in pain. An April 2017 paper published in Drug and Alcohol Dependence charts the historical course of this movement.
In the mid-to-late 1980s, palliative care specialists began to advocate for the use of opioids in cancer patients. At the same time, the World Health Organization created cancer-pain treatment guidelines, which, for the first time, recommended the use of opioids. The 1986 guidelines reassure patients that they need not worry about dependence: “Psychological dependence is not an issue when strong opioids are taken to relieve cancer pain.”
Next came the American Pain Society’s “Pain, the Fifth Vital Sign,” campaign in 1996. The Society aimed to elevate the importance of pain assessment and measurement to the status of the other four vital signs — pulse, temperature, respiration rate, and blood pressure.
“If pain were assessed with the same zeal as other vital signs are, it would have a much better chance of being treated properly,” said APS president James Campbell in his presidential address to the Society on November 11, 1996. “We need to train doctors and nurses to treat pain as a vital sign.”
The campaign’s first major success came in 1999, when the Veteran’s Health Administration adopted pain as the fifth vital sign.
By 2001, the Joint Commission on the Accreditation of Healthcare Organizations said that to maintain accreditation hospitals had to incorporate pain assessment and treatment into patients’ care.
“When the Joint Commission and the Veterans Health Administration declared pain as the fifth vital sign, I think that pain control became a really important metric that a lot of U.S. hospitals started tracking, in terms of trying to improve their patient satisfaction scores and national ranking,” explains Hollie Power, a plastic surgeon at the University of Alberta.
But the practice of medicine didn’t always have this emphasis.
Jason Doctor points to a landmark 1946 study published in Annals of Surgery, “Pain in Men Wounded in Battle,” which analyzes the treatment of 225 soldiers on the European front in World War II.
“That paper, prior to OxyContin, was a popular paper that people would read to understand that pain has a huge psychological component to it, and that the drugs really play a much smaller role,” Doctor says.
“Three-quarters of badly wounded men, although they have received no morphine for a matter of hours, have so little pain that they do not want pain relief medication, even though the questions raised remind them that such is available for the asking,” writes the author of that study, the late anesthesiologist Henry K. Beecher.
Beecher continues: “The man in shock complains far less frequently of wound pain than he does of the great distress produced by thirst.”
Marketing OxyContin: plush toys and free meals
In 1995, Purdue Pharma introduced OxyContin, an extended-release form of the opioid oxycodone.
Then the sales push began.
Over just one year — 2001 — Purdue spent $200 million to promote OxyContin.
In the first six years it was on the market, Purdue spent around six to 12 times more to promote OxyContin than it had promoting one of its predecessors, MS Contin, another long-lasting opioid, writes Art Van Zee in a paper in the American Journal of Public Health from February 2009.
The paper describes how health care providers received branded promotional items including OxyContin plush toys, music CDs and apparel.
Part of the marketing strategy involved influencing medical education programs. Purdue funded over 20,000 pain-related educational programs, such as clinical teaching presentations at hospitals, seminars at conferences and continuing medical education opportunities for doctors, from 1996 to July 2002, according to Van Zee’s paper.
Purdue sought to liberalize the prescribing of opioids for non-cancer related pain, and succeeded. “You won’t believe how committed I am to make OxyContin a huge success,” Richard Sackler, former chair and president of Purdue Pharma wrote in a May 29, 1999, email released in an unsealed court deposition. “It is almost that I dedicated my life to it.”
In just five years OxyContin prescriptions for non-cancer pain increased by nearly tenfold. There were 670,000 such prescriptions in 1997. By 2002, there were 6.2 million.
The relationship between marketing and prescribing
Research suggests that physicians targeted with marketing from pharmaceutical companies prescribe opioids at higher rates than doctors not exposed to marketing.
Several studies use Centers for Medicare and Medicaid Services’ Open Payments data, which tracks payments made by drug and medical device companies to physicians, to analyze how relationships between physicians and drug companies are linked to prescriptions written.
These studies define opioid-related payments as including cash payments, such as speaking fees associated with promoting a drug, and payments-in-kind, such as free meals provided by pharmaceutical representatives to doctors’ offices. These studies find that physicians who receive opioid-related payments tend to prescribe more opioids.
A study in PLoS One from December 2018 looks at physicians who received opioid-related payments, some in 2014 and some in 2015 compared with peers who never received such payments. The authors find that physicians who received opioid-related payments had a larger increase in the number of daily doses of opioids dispensed, as well as in total opioid expenditures, prescribing pricier opioids per dose.
The findings suggest that direct marketing to physicians provides a healthy return on investment, says Mark Zezza, director of policy and research at the New York State Health Foundation and the paper’s lead author. For every dollar spent marketing opioids to physicians, there was a nearly $25 increase in opioid purchases.
“We also found the more payments they receive, the higher those increases were in opioid prescribing. Another key finding is that not only were they prescribing more opioids, but they were generally prescribing more expensive drugs. So, perhaps they were moving towards more brand name drugs,” Zezza says.
“It does kind of sound the alarm that these relationships between drug manufacturers and physicians should be more carefully monitored,” he adds. “Especially for drugs that are highly addictive, and have the potential to cause real harm to patients.”
Another study looking at the same data offers further detail. The study, published in Addiction in June 2019, focuses on 865,347 physicians across the country who filled prescriptions for Medicare patients from 2014 to 2016. “Prescribers who received opioid-specific payments prescribed 8,784 opioid daily doses per year more than their peers who did not receive any such payments,” the authors write.
Payments had the effect of increasing prescriptions for the specific opioid with which they were associated. Hydrocodone-related payments were linked to 5,161 additional daily doses of hydrocodone, oxycodone-related payments were linked to 3,624 additional daily doses of oxycodone, and fentanyl-specific payments were linked to 1,124 additional daily doses of fentanyl compared with peers who did not receive such payments. Additionally, among those who received opioid-related payments, a 1% increase in the amount received was linked to an additional 50 daily doses of opioids.
A series of recent studies further investigate the relationship between pharmaceutical marketing and physicians’ prescribing habits. These studies also use the Open Payments database to look at trends from 2013 to 2016. They were led by Scott Hadland, a pediatrician and addiction specialist at Boston Medical Center and Boston University School of Medicine.
The first study, published in the American Journal of Public Health in September 2017, establishes the extent to which pharmaceutical companies pay physicians to market opioid products. The researchers found that 375,266 non-research opioid-related payments were made to 68,177 physicians over the study period. The authors estimate that about one in 12 physicians in the U.S. received a payment from pharmaceutical companies promoting their opioid medications during the 29-month study period. The bulk of the money spent went to speaking fees or honoraria, but the most common expense was food and beverage payments – 352,298 payments totaling $7,872,581.
“I know that physicians have the opportunity to say no to marketing, but I think in many places, these marketing practices are normalized and felt to be just sort of a part of everyday clinical practice,” Hadland says.
Building on that finding, Hadland looked at how those payments were linked to subsequent prescription trends in a follow-up paper published in JAMA Internal Medicine in June 2018. Using Medicare prescribing data, the authors began with opioid prescriptions filled for Medicare beneficiaries in 2015, and then identified physicians who had written at least 10 opioid prescriptions that year. The authors then analyzed the payments those high-prescribing physicians had received through opioid-related marketing, and looked to see whether there were changes in prescriptions written compared with the previous year.
“Whereas physicians receiving no opioid-related payments had fewer opioid claims in 2015 than in 2014, physicians receiving such payments had more opioid claims,” the authors write. “In multivariable modeling, receipt of any opioid-related payments from industry in 2014 was associated with 9.3% more opioid claims in 2015 compared with physicians who received no such payments.”
Further, the authors found that each additional meal physicians received from a drug company was linked to a subsequent increase in opioid prescriptions.
“Physicians are human,” Hadland says. “Humans are subject to marketing — often in ways that they don’t perceive that marketing can change our behavior in very subtle ways — which, on the level of a single physician may not matter that much. But if you look at marketing widespread across the entire country, these changes in prescribing behaviors really add up.”
Hadland’s final paper in the series geographically links opioid marketing and opioid-related overdose mortality. The paper, published in JAMA Network Open in January 2019, analyzes county-level prescription opioid overdose deaths and county-level opioid marketing payments.
The authors found that mortality from prescription opioid overdoses increased with each standard deviation increase in opioid marketing as measured by dollars spent per capita, number of payments to physicians per capita and number of physicians receiving payments per capita. “Opioid prescribing rates also increased with marketing,” the authors write. They offer that the higher prescription rate might be why overdose deaths increased.
Potential solutions
Because physicians are gatekeepers to highly addictive prescription drugs, researchers have studied ways to moderate prescribing such that opioids are given in limited quantities and only when needed.
One area where researchers agree physicians in the U.S. could stand to benefit is in education around opioid prescribing.
A survey of 162 plastic surgery trainees in the U.S. and Canada published in Plastic and Reconstructive Surgery in July 2019 highlights the issue. Respondents were asked about their opioid prescribing education, factors contributing to their prescribing practices and what they would prescribe for eight different procedures. The authors found that, of the 162 respondents, only 25% of U.S. plastic surgery trainees received opioid-prescriber education, compared with 53% of Canadian trainees. For all but one of the eight procedures in question, U.S. physicians prescribed significantly more morphine milligram equivalents than their Canadian counterparts. Morphine milligram equivalents is a measure that standardizes the strength of opioids by the amount of morphine that would be needed to achieve the same pain-relieving effect.
Both U.S. and Canadian trainees were unlikely to routinely offer their patients preoperative counseling on the use of opioids.
“Health care delivery is very different between the U.S. and Canada,” explains Hollie Power, the lead author of the study. “Obviously, nobody wants our patients to suffer. But it [pain management] became an important metric in quality [in the U.S.] as well. That’s not so much of a consideration in Canada, where healthcare is government funded, and patients don’t tend to look at hospital rankings or things like that.”
Power notes that Canada has had tighter regulations in effect for decades around opioid prescribing.
“We need to be educating our providers to be able to know what they need to prescribe,” Power says. “Another gap that we identified was that there doesn’t exist guidelines in our literature for how much medication do patients actually require for procedures that we’re doing commonly.” She suggests a multi-faceted approach, combining both prescriber education initiatives and policies to limit quantities of opioids prescribed.
Here’s what that might look like in practice.
In October 2017, the Michigan Opioid Prescribing Engagement Network released opioid prescribing guidelines for nine surgical procedures to clinicians participating in the Michigan Surgical Quality Collaborative, a statewide initiative to improve surgical care.
Researchers compared opioid prescribing before and after these guidelines were released, analyzing data from 11,716 patients across 43 hospitals collected from February 2017 to May 2018. They found that prescriptions declined on average from 26 pills to 18 pills after the guidelines were released.
Patients also took fewer of the pills they were prescribed. As measured by patient-reported survey data, opioid consumption in the post-operative period dropped from 12 pills to nine pills, “possibly as a result of patients anchoring and adjusting their expectations for opioid use to smaller prescriptions,” the authors of the August 2019 New England Journal of Medicine publication explain. Although patients received smaller prescriptions and used fewer pills after the guidelines were published, the researchers found no clinically important changes in the patients’ satisfaction and pain scores.
Similar to the study of Michigan’s opioid prescribing guidelines is a February 2018 study in the American Journal of Emergency Medicine tracking the effects of an emergency department opioid prescribing policy. The policy resulted in declines in opioid prescriptions. Compared with the control emergency department, the two intervention hospitals had a more pronounced decline in opioid prescribing. The authors conclude that emergency department-based policies might help reduce opioid prescribing.
Prescription drug monitoring programs, which allow physicians to view patients’ prescription history in order to avoid overprescribing or illegitimate prescribing of opioids, and which have been implemented in many states, have proven less effective. A January 2018 study of national data published in Addictive Behaviors finds that there were not significant differences in the likelihood that physicians would prescribe opioids for non-cancer chronic pain between states with prescription drug monitoring programs and those without.
An August 2018 study published in Science attempts to make the relationship between opioid prescribing and overdose deaths more visible to physicians. The intervention was simple: a letter from the county’s medical examiner explaining to the prescribing physicians that their patient had subsequently died of an opioid overdose. The authors conducted a randomized trial of 861 physicians whose patients overdosed. The intervention group received the letter, which included a safe prescribing warning consisting of these recommendations:
- Avoid co-prescribing an opioid and a benzodiazepine.
- Minimize opioid prescribing for acute pain
- Taper long-term users off opioids
- Avoid prescriptions lasting for three consecutive months or longer and prescribe naloxone, an opioid overdose antidote.
The control group received no communication.
Physicians in the intervention group decreased their opioid prescribing by 9.7% — as measured by milligram morphine equivalents in prescriptions filled — in the three months after the letter was sent. These physicians also started fewer patients on opioids and wrote fewer high-dose prescriptions than the control group.
“I think physicians believe there is an opioid epidemic, but they think it’s not happening in their clinic, right? It’s happening elsewhere, there are these bad doctors,” says Jason Doctor, lead author of the study. “They can watch the news and see all the terrible things happening and believe that this is happening, but think they’re not part of the problem.”
He continues, “You can be a bad actor without knowing you’re a bad actor. You can be influenced to do these things through marketing campaigns and believing that pain can be knocked out with opioids.”
The aftermath
Overprescribing is a cause. Addiction is an effect.
As physicians attempt to address opioid use disorder, the focus has turned to evidence-based treatment, such as medication-assisted therapy. Methadone, buprenorphine and naltrexone are examples of medication-assisted therapy for opioid use disorder. These medications reduce symptoms of craving and withdrawal. Under the Drug Addiction Treatment Act of 2000, physicians must complete a training program if they want to prescribe medication-assisted therapy outside of an opioid treatment program.
Ironically, the very marketing tactics that contributed to America’s opioid crisis might also contribute to the solution. A September 2019 study in the Journal of Substance Abuse Treatment finds that physicians who received payments from pharmaceutical companies as part of marketing efforts for medications that treat opioid use disorder prescribed 1,080 daily doses more of these medications each year than their peers who received no such payments.
“We’ve got a problem in this country right now with the sort of weird policies where it’s harder to prescribe buprenorphine to treat an opioid addiction than it is to prescribe the opioid in the first place,” says David Bradford, senior author and chair in public policy at the University of Georgia. “You don’t need extra training to prescribe an opioid, but if you want to prescribe a medicine to help people get off opioid addiction, you do need it. And so, how do we overcome that barrier? Well, it may be that pharmaceutical marketing helps you in that.”
The image at the top of this article was obtained from the Flickr account of Cindy Shebley and is being used under a Creative Commons license. No changes were made.
