
A few decades ago, at least in North America, Lyme disease affected only small parts of New England and the upper Midwest. But the tiny ticks that carry the debilitating illness have spread rapidly in recent years, establishing themselves in twice as many American counties and pushing 46 kilometers deeper into Canada every year.
The reason, a 2016 U.S. government report affirmed with “high confidence,” is climate change.
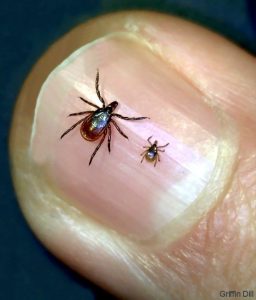
Named for the woodsy Connecticut town where it was first identified in the late 1970s, Lyme disease is caused by the bacteria Borrelia burgdorferi, which is carried by blacklegged ticks. Also known as deer ticks, these tiny arachnids are not infested when they hatch. Instead, ticks pick up the germ when feeding on the blood of small mammals such as chipmunks and white-footed mice. Adult ticks and nymphs (adolescents about the size of a poppy seed) then spread the Lyme-causing pathogens by clinging to these animals, as well as deer and birds. Removing a tick within 24 hours usually prevents it from passing on the bacteria to humans, according to the Centers for Disease Control and Prevention (CDC). More than half of ticks do not carry the disease.
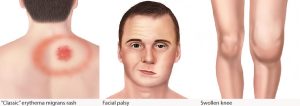
Lyme can cause fever, skin rashes, fatigue, arthritis-like joint pain and, in a small number of cases, brain inflammation or heart problems. The illness is usually treatable with antibiotics, but sometimes symptoms persist for years — a condition often known as chronic Lyme disease, though doctors prefer post-treatment Lyme disease syndrome (PTLDS). A 2014 survey in Public Health found widespread uncertainty about the causes or treatment for PTLDS among professors of public health.
Lyme is the most common disease transmitted by insects in the U.S. The CDC counts about 30,000 confirmed cases per year, but estimates about 300,000 cases overall, many of them untreated. It is also found across temperate regions of Europe and Asia. (Though only recognized for about 40 years, Lyme is not a modern phenomenon. A 5,300-year-old man found frozen in the Italian Alps in 1991 is the oldest known human to have carried the bacteria that causes Lyme, National Geographic reported in 2011.)
So how is climate change a factor?
Ticks are delicate little bugs. They don’t like freezing or getting too dry. Mild winters help them survive; their eggs hatch sooner, lengthening the feeding and molting season. Deer help them move further north into areas where cooler temperatures would have once killed them. “Deer ticks are mostly active when temperatures are above 45 degrees Fahrenheit, and they thrive in areas with at least 85 percent humidity. Thus, warming temperatures associated with climate change are projected to increase the range of suitable tick habitat and are therefore one of multiple factors driving the observed spread of Lyme disease,” according to the Environmental Protection Agency, which sees the spread of Lyme as an indicator of climate change.
Diagnoses and treatment
Lyme can be difficult to diagnose because ticks often go undetected (a nymph is only about 2 millimeters wide) and its symptoms mimic other conditions such as the flu. One of the most telling signs is a bull’s-eye-shaped rash. The National Institutes of Health (NIH) has a resource page on prevention and diagnosis.
There was a vaccine available until 2002, when the manufacturer, facing criticism that the effect wore off over time, discontinued production. Scientists are again researching a vaccine, some sponsored by the NIH.
In addition to the CDC and the NIH, other U.S. government agencies with resource pages on Lyme include the Department of Agriculture, the National Library of Medicine and the Connecticut Department of Health. In Canada, the Public Health Agency has resources including numbers of cases and the changing risk factors. The National Health Service in Great Britain has a list of ongoing clinical trials.
Research
“Epidemiology of Lyme Disease”
Mead, Paul. Infectious Disease Clinics of North America, 2015. DOI: 10.1016/j.idc.2015.02.010.
Abstract: “Lyme disease is the most common vector-borne illness in North America and Europe. The etiologic agent, Borrelia burgdorferi sensu lato, is transmitted to humans by certain species of Ixodes ticks, which are found widely in temperate regions of the Northern hemisphere. Clinical features are diverse, but death is rare. The risk of human infection is determined by the geographic distribution of vector tick species, ecologic factors that influence tick infection rates, and human behaviors that promote tick bites. Rates of infection are highest among children 5 to 15 years old and adults older than 50 years.”
“Lyme Disease Testing by Large Commercial Laboratories in the United States”
Hinckley, Alison; et al. Clinical Infectious Diseases, 2014. DOI: 10.1093/cid/ciu397.
Abstract: “Large commercial laboratories in the United States were surveyed to determine Lyme disease testing frequency, practices, and results. Approximately 3.4 million tests were conducted in 2008; 62 percent in accordance with recommendations. We estimate that 288,000 infections occurred among 2.4 million patients from whom samples were submitted.”
“Accelerated Phenology of Blacklegged Ticks Under Climate Warming”
Levi, Taal; et al. Philosophical Transactions of the Royal Society, 2015. DOI: 10.1098/rstb.2013.0556.
Abstract: “The phenology of tick emergence has important implications for the transmission of tick-borne pathogens. A long lag between the emergence of tick nymphs in spring and larvae in summer should increase transmission of persistent pathogens by allowing infected nymphs to inoculate the population of naive hosts that can subsequently transmit the pathogen to larvae to complete the transmission cycle. In contrast, greater synchrony between nymphs and larvae should facilitate transmission of pathogens that do not produce long-lasting infections in hosts. Here, we use 19 years of data on blacklegged ticks attached to small-mammal hosts to quantify the relationship between climate warming and tick phenology. Warmer years through May and August were associated with a nearly three-week advance in the phenology of nymphal and larval ticks relative to colder years, with little evidence of increased synchrony. Warmer Octobers were associated with fewer larvae feeding concurrently with nymphs during the following spring. Projected warming by the 2050s is expected to advance the timing of average nymph and larva activity by 8-11 and 10-14 days, respectively. If these trends continue, climate warming should maintain or increase transmission of persistent pathogens, while it might inhibit pathogens that do not produce long-lasting infections.”
“The Impact of Temperature and Precipitation on Blacklegged Tick Activity and Lyme Disease Incidence in Endemic and Emerging Regions”
Burtis, James; et al. Parasites and Vectors, 2016. DOI: 10.1186/s13071-016-1894-6.
Conclusions: “Recently endemic regions showed an increase in Lyme disease incidence over time, while incidence in long-term endemic regions appears to have stabilized. Only within the stabilized areas were we able to detect reduced Lyme disease incidence in years with hot, dry summer weather. These patterns were reflected in our field data, which showed that questing activity of nymphal I. scapularis was reduced by hot, dry summer weather.”
“Lyme Disease Ecology in a Changing World: Consensus, Uncertainty and Critical Gaps for Improving Control”
Kilpatrick, A. Marm; et al. Philosophical Transactions of the Royal Society, 2017. DOI: 10.1098/rstb.2016.0117.
Abstract: “Lyme disease is the most common tick-borne disease in temperate regions of North America, Europe and Asia, and the number of reported cases has increased in many regions as landscapes have been altered. Although there has been extensive work on the ecology and epidemiology of this disease in both Europe and North America, substantial uncertainty exists about fundamental aspects that determine spatial and temporal variation in both disease risk and human incidence, which hamper effective and efficient prevention and control. Here we describe areas of consensus that can be built on, identify areas of uncertainty and outline research needed to fill these gaps to facilitate predictive models of disease risk and the development of novel disease control strategies. Key areas of uncertainty include: (i) the precise influence of deer abundance on tick abundance, (ii) how tick populations are regulated, (iii) assembly of host communities and tick-feeding patterns across different habitats, (iv) reservoir competence of host species, and (v) pathogenicity for humans of different genotypes of Borrelia burgdorferi. Filling these knowledge gaps will improve Lyme disease prevention and control and provide general insights into the drivers and dynamics of this emblematic multi-host-vector-borne zoonotic disease.”
“Estimated Effects of Projected Climate Change on the Basic Reproductive Number of the Lyme Disease Vector Ixodes scapularis”
Ogden, Nicholas; et al. Environmental Health Perspectives, 2014. DOI:10.1289/ehp.1307799.
Conclusions: “Climate warming may have co-driven the emergence of Lyme disease in northeastern North America, and in the future may drive substantial disease spread into new geographic regions and increase tick-borne disease risk where climate is currently suitable. Our findings highlight the potential for climate change to have profound effects on vectors and vector-borne diseases, and the need to refocus efforts to understand these effects.”
“Does High Biodiversity Reduce the Risk of Lyme Disease Invasion?”
Bouchard, Catherine; et al. Parasites and Vectors, 2013. DOI: 10.1186/1756-3305-6-195.
Conclusions: “I. scapularis abundance and B. burgdorferi prevalence varied with explanatory variables in patterns consistent with the known biology of these species in general, and in the study region in particular. The evidence for a negative effect of host biodiversity on I. scapularis invasion was mixed. However, some evidence suggests that community biodiversity beyond just host diversity may have direct or indirect inhibitory effects on parasite invasion that warrant further study.
“Lyme Disease and Post-Treatment Lyme Disease Syndrome: The Neglected Disease in Our Own Backyard”
Crowder, L.A.; et al. Public Health, 2014. DOI: 10.1016/j.puhe.2014.06.016.
“Objectives: A survey was developed to assess experience and opinions about Lyme disease and post-treatment Lyme disease syndrome (PTLDS) among faculties in public health. No previous surveys of public health faculties have been found in the literature. […] Results: A 15 percent response rate was seen for the survey. 50 percent of respondents were from Lyme endemic states. Less than 5 percent of faculty members consider themselves expert in Lyme or PTLDS. Many faculty members had known someone with Lyme disease or PTLDS, but few had been diagnosed themselves. Most believe that PTLDS can be severe and chronic, is not easy to treat, and does not resolve on its own, but were uncertain about its etiology. Most respondents also felt that the incidence of Lyme disease will increase and that more education is needed.”
“Predicting the Speed of Tick Invasion: An Empirical Model of Range Expansion for the Lyme Disease Vector Ixodes Scapularis in Canada”
Leighton, Patrick A.; et al. Journal of Applied Ecology, 2012. DOI: 10.1111/j.1365-2664.2012.02112.x.
Abstract: “By tracking I. scapularis invasion in Canada over the past two decades, we show that I. scapularis is rapidly expanding its range and is likely to colonize the most densely populated areas of southern Canada in the coming decade. These projections suggest that prompt action is necessary to prepare the Canadian public for a likely epidemic of Lyme disease, with emphasis on focusing surveillance activities to confirm the locations of emerging Lyme disease risk.”
“County-Scale Distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the Continental United States”
Eisen, Rebecca; Eisen, Lars; Beard, Charles. Journal of Medical Entomology, 2016. DOI: 10.1093/jme/tjv237.
Abstract: “Since 1991, when standardized surveillance and reporting began, Lyme disease case counts have increased steadily in number and in geographical distribution in the eastern United States. Similar trends have been observed for anaplasmosis and babesiosis. To better understand the changing landscape of risk of human exposure to disease agents transmitted by I. scapularis and I. pacificus, and to document changes in their recorded distribution over the past two decades, we updated the distribution of these species from a map published in 1998. The presence of I. scapularis has now been documented from 1,420 (45.7 percent) of the 3,110 continental United States counties, as compared with 111 (3.6 percent) counties for I. pacificus. Combined, these vectors of B. burgdorferi and other disease agents now have been identified in a total of 1,531 (49.2 percent) counties spread across 43 states. This marks a 44.7 percent increase in the number of counties that have recorded the presence of these ticks since the previous map was presented in 1998, when 1,058 counties in 41 states reported the ticks to be present. Notably, the number of counties in which I. scapularis is considered established (six or more individuals or one or more life stages identified in a single year) has more than doubled since the previous national distribution map was published nearly two decades ago.”
“Is There a Lyme-Like Disease in Australia? Summary of the Findings to Date”
Chalada, Melissa Judith; Stenos, John; Bradbury, Richard Stewart. One Health, 2016. DOI: 10.1016/j.onehlt.2016.03.003.
Abstract: “Lyme Borreliosis is a common tick-borne disease of the northern hemisphere caused by the spirochaetes of the Borrelia burgdorferi sensu lato (B. burgdorferi s. l.) complex. It results in multi-organ disease with arthritic, cardiac, neurological and dermatological manifestations. In the last 25 years there have been over 500 reports of an Australian Lyme-like syndrome in the scientific literature. However, the diagnoses of Lyme Borreliosis made in these cases have been primarily by clinical presentation and laboratory results of tentative reliability and the true cause of these illnesses remains unknown. A number of animals have been introduced to Australia that may act as B. burgdorferi s. l. reservoirs in Lyme-endemic countries, and there are some Australian Ixodes spp. and Haemaphysalis spp. ticks whose geographical distribution matches that of the Australian Lyme-like cases. Four published studies have searched for Borrelia in Australian ticks, with contradicting results. The cause of the potential Lyme-like disease in Australia remains to be defined. The evidence to date as to whether these illnesses are caused by a Borrelia species, another tick-borne pathogen or are due to a novel or unrelated etiology is summarized in this review.”
“Modeling the Geographic Distribution of Ixodes Scapularis and Ixodes Pacificus (Acari: Ixodidae) in the Contiguous United States”
Hahn, Micah; et al. Journal of Medical Entomology, 2016. DOI: 10.1093/jme/tjw076.
Abstract: “In addition to serving as vectors of several other human pathogens, the black-legged tick, Ixodes scapularis, and western black-legged tick, Ixodes pacificus, are the primary vectors of the spirochete (Borrelia burgdorferi) that causes Lyme disease, the most common vector-borne disease in the United States. Over the past two decades, the geographic range of I. pacificus has changed modestly while, in contrast, the I. scapularis range has expanded substantially, which likely contributes to the concurrent expansion in the distribution of human Lyme disease cases in the Northeastern, North-Central and Mid-Atlantic states. Identifying counties that contain suitable habitat for these ticks that have not yet reported established vector populations can aid in targeting limited vector surveillance resources to areas where tick invasion and potential human risk are likely to occur. We used county-level vector distribution information and ensemble modeling to map the potential distribution of I. scapularis and I. pacificus in the contiguous United States as a function of climate, elevation, and forest cover. Results show that I. pacificus is currently present within much of the range classified by our model as suitable for establishment. In contrast, environmental conditions are suitable for I. scapularis to continue expanding its range into northwestern Minnesota, central and northern Michigan, within the Ohio River Valley, and inland from the southeastern and Gulf coasts. Overall, our ensemble models show suitable habitat for I. scapularis in 441 eastern counties and for I. pacificus in 11 western counties where surveillance records have not yet supported classification of the counties as established.”
“Linkages of Weather and Climate with Ixodes Scapularis and Ixodes Pacificus (Acari: Ixodidae), Enzootic Transmission of Borrelia Burgdorferi, and Lyme Disease in North America”
Eisen, Rebecca; et al. Journal of Medical Entomology, 2016. DOI: 10.1093/jme/tjv199.
Abstract: “Lyme disease has increased both in incidence and geographic extent in the United States and Canada over the past two decades. One of the underlying causes is changes during the same time period in the distribution and abundance of the primary vectors: Ixodes scapularis and Ixodes pacificus in eastern and western North America, respectively. Aside from short periods of time when they are feeding on hosts, these ticks exist in the environment where temperature and relative humidity directly affect their development, survival, and host-seeking behavior. Other important factors that strongly influence tick abundance as well as the proportion of ticks infected with the Lyme disease spirochete, Borrelia burgdorferi, include the abundance of hosts for the ticks and the capacity of tick hosts to serve as B. burgdorferi reservoirs. Here, we explore the linkages between climate variation and: 1) duration of the seasonal period and the timing of peak activity; 2) geographic tick distributions and local abundance; 3) enzootic B. burgdorferi transmission cycles; and 4) Lyme disease cases. We conclude that meteorological variables are most influential in determining host-seeking phenology and development, but, while remaining important cofactors, additional variables become critical when exploring geographic distribution and local abundance of ticks, enzootic transmission of B. burgdorferi, and Lyme disease case occurrence. Finally, we review climate change-driven projections for future impact on vector ticks and Lyme disease and discuss knowledge gaps and research needs.”
